Protective layer
According to the DIN EN ISO 8044 standard, the protective layer is a layer of a substance on a metal surface that decreases the corrosion rate. It may either be formed by coating or by change of the surface material composition (cf. DIN 8580). Possible protective layers include surface, passive and diffusion layers, or protective layers formed by lime and rust.
Surface layer
- This is a layer of solid reaction products formed by corrosion which covers the surface more or less uniformly. It allows slowing down corrosion processes.
- If the surface layer forms unevenly, corrosion cells may develop.
- A surface layer is only regarded as a protective layer if it is uniformly developed and slows down corrosion substantially. See Fig. 1 Protective layer
Passive layer
- This is a protective layer created by corrosion often not detectable by an optical microscope. It renders the metal "passive" to its environment (see DIN EN ISO 8044).
- The best-known passive layers are those on stainless steels, aluminium and titanium.
Diffusion layer
- A diffusion layer is a layer formed when a metal or non-metal diffuses into the base material.
Lime-rust protective layer
- The formation of a lime-rust protective layer depends on factors such as pH value, oxygen content, salt content, temperature, flow velocity, water hardness etc.
- All naturally occurring water contains carbonic acid, both in its free form and in the form of anions. The chemically bound form is found as either calcium carbonate or magnesium carbonate.
- The free carbon dioxide (CO2) is mostly found as dissolved gas. The free carbonic acid ensures that the hydrogen carbonates are held in solution according to the lime-carbonic acid equilibrium.
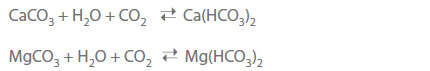
- The portion of free carbonic acid contained in water in excess of the concentration of associated free carbonic acid is called excess or aggressive carbonic acid.
- Only waters which do not contain excess free carbonic acid may form surface layers on metals. With regard to unalloyed steel, this means that after initial rust attacks corrosion is considerably reduced due to the lime-rust protective layer (e.g. limescale).
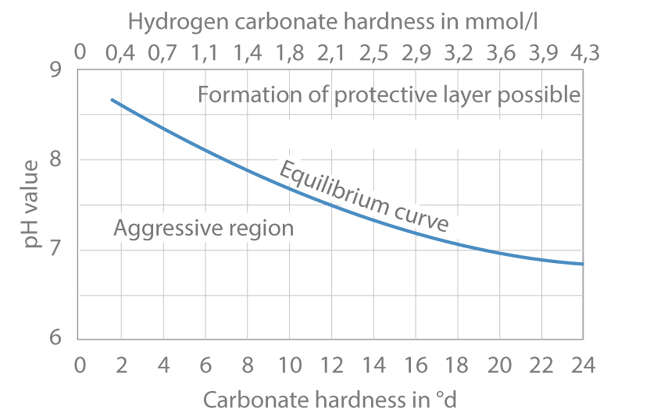
Fig. 1 Protective layer: Equilibrium curve for the formation of surface layers as a function of the carbonate hardness and the pH value
